Hydrogen Induced Cracking
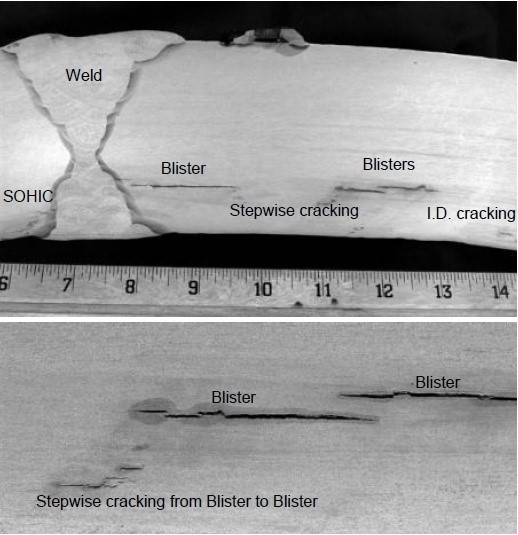
Hydrogen Induced Cracking (HIC) is a common form of wet H2S cracking caused by the blistering of a metal due to a high concentration of hydrogen. The blistering damage tends to form parallel to the surface and to the direction of hoop stress.
HIC usually occurs due to the effects of aqueous hydrogen charging of steel in wet Hydrogen Sulphide (H2S) refinery process environments. It can occur at relatively low temperatures, largely as a result of atomic hydrogen from wet H2S corrosion reactions which enter the steel and collect at inclusions or impurities within the steel. The H2S prevents the hydrogen recombination reaction that would normally occur so, rather than bubbling off from the corroding surface, the hydrogen atoms are forced into the metal structure causing corrosion and weakness.

The damage occurs when the hydrogen collects at inclusions or impurities in the steel. It is manifested as blisters or blister cracks oriented parallel to the plate surface. Once the ductility of the metal has reduced to a significant amount, the metal will form stepwise internal cracks connecting adjacent hydrogen blisters.
The inspection requires characterization of the defect areas to differentiate between spot inclusions, laminations, and different stages of hydrogen Induced cracking (HIC)
Type of Defects
- Spot inclusions
- Laminar Inclusions
- Laminar Blistering
- Blistering
- Stepwise cracking
At Focus Integrity Solutions we have all the required expertises along with needed equipment to differentiate and distingusigh between the different stages of damage. This helps our clients to take decision on repair and replacement.
Below mentioned techniques are used for detection and differentiate between the different stages of damage.
- PAUT
- TOFD